Myth #8
Ageing of heart is irreversible.
False! There is now very new and emerging evidence that this assumption long held over decades, is untrue. Unlike a broken vase that can’t be fixed, our heart seems to have the capability of acting differently! Ageing of the heart can cause many effects such as stiffness of the left ventricle, thickening of arteries and capillaries walls which eventually increases the risk of heart diseases.
MITOCHONDRIAL TARGETING:
Mitochondria generates energy for the cell but in the process it also accumulates reactive oxygen species (ROS). Too much of reactive oxygen species damage mitochondria DNA and protein causing mitochondria dysfunction, which play a critical role in ageing of heart.
As the heart requires a lot of energy, heart cells are full of mitochondria that are needed to produce the energy that the heart needs. Hence, the heart is very prone to oxidative damage and mitochondria dysfunction.
Old mice that were created with overexpressed mitochondrial-targeting catalase were found to have reduced left ventricular thickening and better function, despite ageing.
We know that there are pros and cons to everything and the same applies for this treatment. Although reactive oxygen species can cause damage, they also play a useful role in hormones, signalling, etc. Hence, exposing young mice to this treatment can cause an imbalance and lead to them expressing “older” sets of protein. Therefore this treatment may need to be fine-tuned in proper doses, adjusting it to the age of the patient.
So what can we do? How can we prevent mitochondria dysfunction from happening?
Fret not! A study just found that mitochondrial-targeting catalase therapy helps to reduce mitochondria reactive oxygen species and hence reduce ageing of heart. You can think of the catalase like a scissors which cuts away the reactive oxygen species (specifically hydrogen peroxide), reducing the amount of reactive oxygen species.
Created using Flaticon
Created using Flaticon
Created using Flaticon
Created using Flaticon & Microsoft PPT
Created using Flaticon & Microsoft PPT





OSKM FRAGMENTS:
We know that as we age, the risk of heart damage becomes more prominent.
From the image, we can see that a fundamental issue with the heart is that adult heart cells cannot regenerate and damaged cells cannot be replaced. We need the less mature (“young”) cells for regeneration!
Created using Flaticon & Microsoft PPT
But what are mature heart cells?



Created using Microsoft PPT
Mature cells refer to cells that have differentiated to carry out specific functions. How then can we turn back time and switch the mature cells to less mature (“young”) ones?
The 4 knights - OSKM Factors
.jpeg)
This is when our 4 knights arrive! They are the OSKM factors, which stands for Oct4, Sox2, Klf4 and c-Myc respectively.
.jpeg)
OSKM are transcription factors that bind to certain genes to switch them on, allowing key proteins to be produced. These key proteins will then help to "unlock the gates" ( signals required for embryonic stem cell properties) that would allow the "old" cells to be “reprogrammed” and be “youthful” again.
The OSKM cocktail therefore acts like an immortal potion, allowing our mature heart cells to rejuvenate. A younger heart cell now means that it is able to divide and multiply, and replace damaged cells!
However we have to be careful! Because long-term treatment with OSKM makes cells revert all the way to stem-cell states, which will grow out of control and cause formation of heart tumours. (Proven in the study on mice).
Immortal potion - What happens when its too much?

Created using Flaticon & Microsoft PPT
Moreover, stem-cells may lose the capability to differentiate back to the cell of origin (heart cells). This means that an overdose of OSKM can cause our heart cells to become non-heart cells, and that's when the heart stops functioning as a heart! This is why when OSKM is given for too long a dose, they start to become increasingly sick and die. When long-term OSKM was used, reversing the process is no longer possible.
Short-term OSKM treatment
Experimentally, it seems that heart cells should only be treated with OSKM for a short term to produce a partially reprogrammed cell that is able to proliferate and still maintain its capability to differentiate back to heart cells. This means that short-term treatment is reversible and early signs of abnormal heart function can be solved!
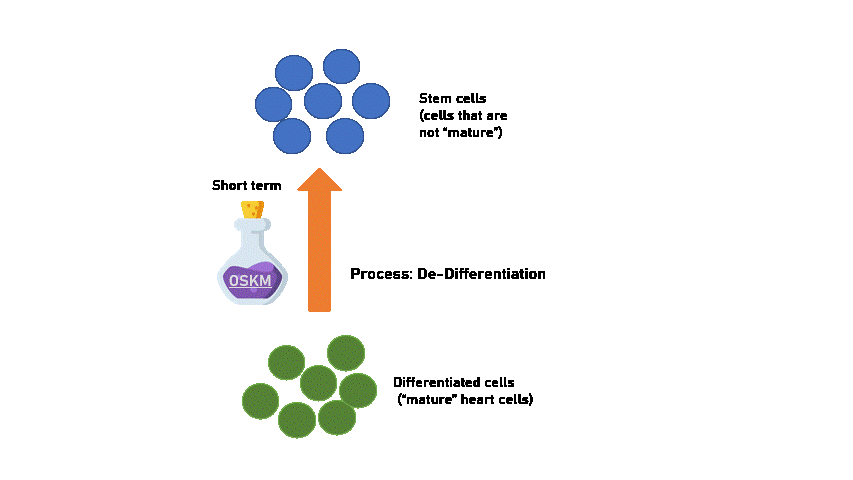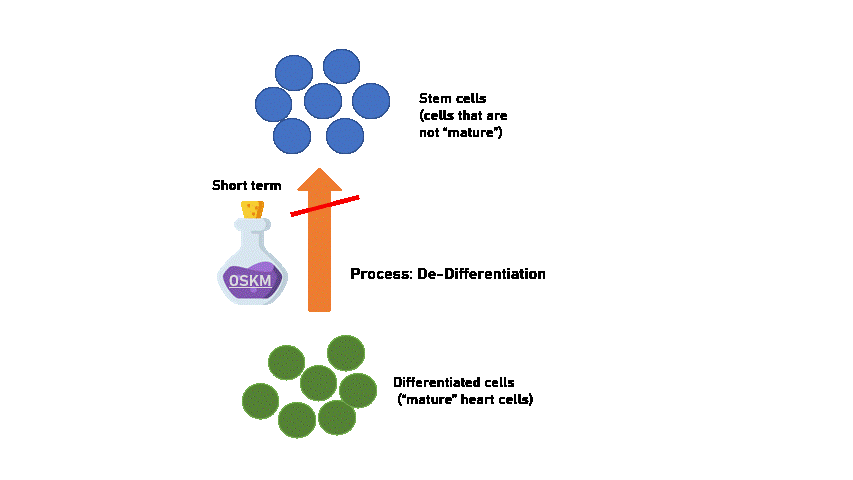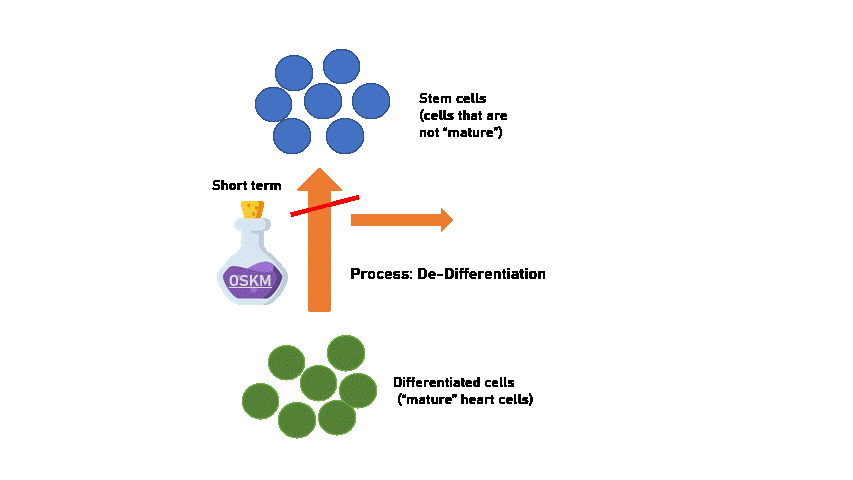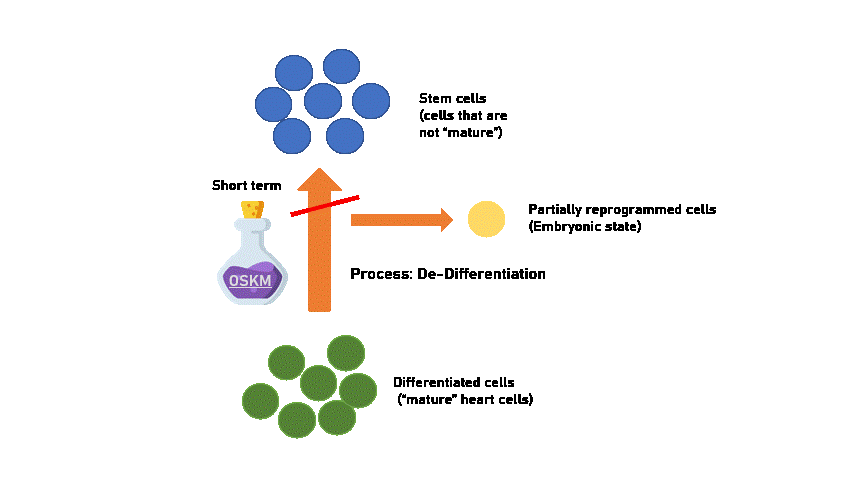
Created using Flaticon & Microsoft PPT
How do we know that OSKM fragments are really our knights? - Experiment
The study induced heart attack on the mice and showed that short-term OSKM expression before and during heart attack helps to reduce heart
damage and improves cardiac function, demonstrating that OSKM is able to facilitate heart regeneration.

Created using Flaticon & Microsoft PPT
Now we know heart damage can be reversible! So we should not feel hopeless if we ever experience any damage to our heart tissue because we have our special scissors (mitochondrial-targeting catalase) as well as our four knights (OSKM factors) to the rescue!
Written by: Christy Liam
References
-
Chen, Y., Lüttmann, F. F., Schoger, E., Schöler, H. R., Zelarayán, L. C., Kim, K.-P., Haigh, J. J., Kim, J., & Braun, T. (2021). Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science, 373(6562), 1537–1540. https://doi.org/10.1126/science.abg5159
-
Dai, D.-F., Johnson, S. C., Villarin, J. J., Chin, M. T., Nieves-Cintrón, M., Chen, T., Marcinek, D. J., Dorn, G. W., Kang, Y. J., Prolla, T. A., Santana, L. F., & Rabinovitch, P. S. (2011). Mitochondrial oxidative stress mediates angiotensin ii–induced cardiac hypertrophy and GΑQ overexpression–induced heart failure. Circulation Research, 108(7), 837–846. https://doi.org/10.1161/circresaha.110.232306
-
Basisty, N., Dai, D. F., Gagnidze, A., Gitari, L., Fredrickson, J., Maina, Y., Beyer, R. P., Emond, M. J., Hsieh, E. J., MacCoss, M. J., Martin, G. M., & Rabinovitch, P. S. (2016). Mitochondrial‐targeted catalase is good for the old mouse Proteome, but not for the young: ‘reverse’ antagonistic pleiotropy? Aging Cell, 15(4), 634–645. https://doi.org/10.1111/acel.12472
-
Zhu, Y., Do, V. D., Richards, A. M., & Foo, R. (2021). What we know about Cardiomyocyte Dedifferentiation. Journal of Molecular and Cellular Cardiology, 152, 80–91. https://doi.org/10.1016/j.yjmcc.2020.11.016